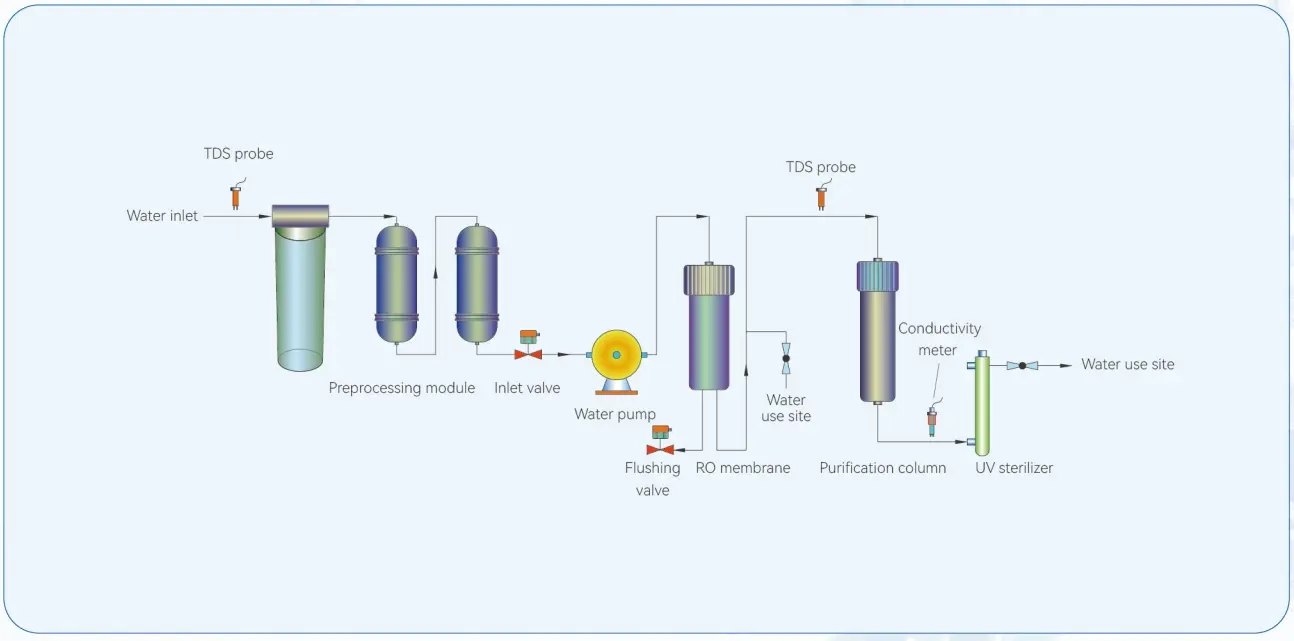
| EQUIPMENT PARAMETERS AND RELEVANT REQUIREMENTS |
| Equipment model | YS-DI-(10-20)AL/H | Water quality standards | GB/T 6682-2008 |
| Débit d'eau | 10L-20L/H |
| Quality of water production |
| Electrical resistivity(25°C),MΩ*cm | ≥15-18MQ·cm |
| Electrical conductivity (25’C), mS/m | 0.005 |
| Absorbency(254nm,1cm optical distance) | 0 |
| Soluble silicon content (as Si02), mg/L | <0.01 |
| Oxidizable substance content (in O), mg/L | <0.08 |
| Evaporation residue content 105’C+2’c, mg/L | 0.6 |
| Membrane desalination rate | 99.60% |
| Additional information |
| Equipment size | 26*43*43cm |
| Influent requirements Water pressure Water temperature Power supply |
| Incoming water pressure | ≥20.2MPa |
| Water temperature | 5-45°C |
| Power supply | AC220V+10% 50HZ,with 24V safety voltage |
| Power supply | (0.1-0.15)kw |
| Scope of application |
| Atomic absorption/fluorescence/laboratory/clinical lab; Fully automatic biochemical/luminescent immune equipment water supply, High-precision electrochemical configuration water/battery cells/storage battery/chip water; Water supply for constant quantitative analysis/medicine, etc. |







LH_WH_600x800px.webp)
